Abstract
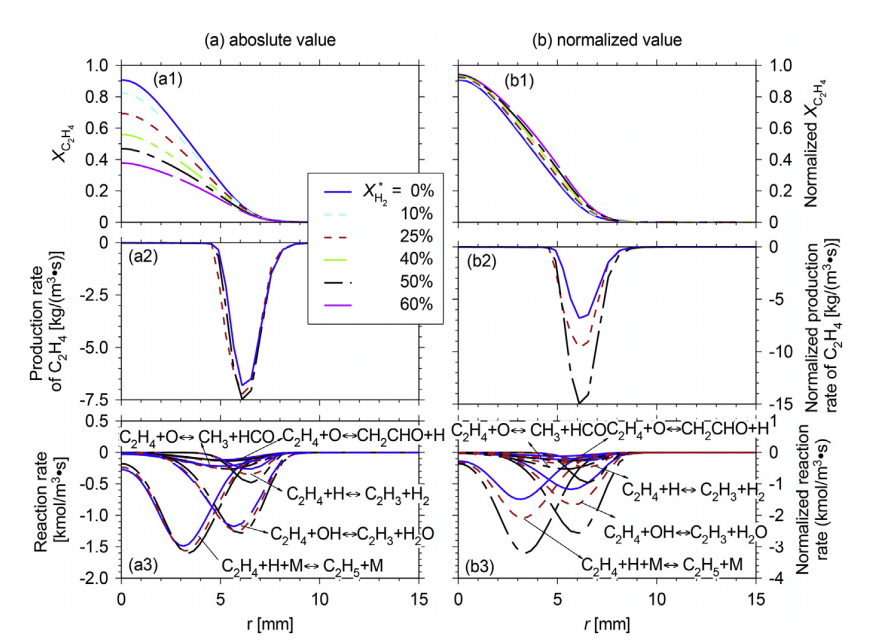
This paper numerically investigated the chemical kinetic effect of hydrogen addition, ranging from 0% to 60% (by vol.), on ethylene jet flames in a hot coflow. The Eddy Dissipation Concept model with the San Diego mechanism was used for all the calculations. To validate the present modeling, four flames were predicted under the experimental conditions of Medwell et al. [Combust. Flame 152 (2008) 100–113] and the predictions are found to agree quite well with the measurements. As the hydrogen content is higher, the jet entrainment and the jet velocity decay are enhanced, whilst the local equivalence ratio in the reaction zone is decreased. Moreover, under MILD condition, the hydrogen addition leads to remarkable increases in the mole fractions of H, O and OH radicals in the reaction zone, which then promotes the oxidation of C2H4 significantly. When hydrogen is added, the increasing rate of H mole fraction is greater than that of OH mole fraction. This hence makes the reactions attacked by H strengthened while those attacked by OH weakened. With respect to those at traditional air condition, the higher-carbon path (C2H4 → C2H3 → C2H2 → C2H, HCCO → CH2CO → CO → CO2) of the C2H4 oxidation at MILD condition becomes more important while the lower-carbon path (C2H4 → [C2H5→]CH3 → [S–CH2, T–CH2, CH3O, CH3OH, and CH2OH] → CH2O → HCO → CO → CO2 and C2H4 → [C2H5→]CH3 → [S–CH2 → T–CH2] → CO → CO2) is weakened. Further, under MILD condition at Xo2 = 3%, the H2 addition weakens the importance of higher-carbon path but enhances that of the lower-carbon path, thus the C2H2 mole fraction is greatly reduced. Considering that C2H2 is an important precursor of soot, the decrease of C2H2 mole fraction and the local equivalence ratio indicate that H2 might have the potential to reduce the soot emission.